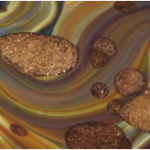
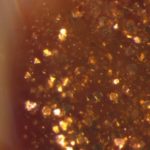
Eleanor Magson looks at the role of chance in scientific discovery and art. The glittering swirls of this 18th-century Venetian bowl give us a perfect opportunity to think about the relationship between the natural world and man-made products, and the complex techniques that glass-makers employ. Eleanor worked on this project during her Masters in Science Communication at Imperial College London.
The Adventures of Making Aventurine
In 16th-century Italy, amid the weaving canals of Murano, a Venetian glass-maker stumbled upon a new technique. Sparkling puddles of gold were forming underneath the surface of pure, clear glass. Legend has it that the inventor of this technique accidentally spilled copper salts into a batch of molten glass. In the low-oxygen environment of the furnace, the copper transformed from a sooty black powder into shimmering golden pools. The glass produced from this technique was named aventurine, from the Italian word aventurra, meaning ‘by chance’. Whether or not the original creator of aventurine glass genuinely made his discovery by accident, the name stuck.
Aventurine from the 16th century looks like it is made of golden glitter, as it is a vitreous (or glassy) mass brought to life with small copper crystals. The reflective crystals twinkle like stars, or ‘stelle’, thus forming the basis of aventurine’s alternative name, stellaria.
Murano has long upheld its crown as the definitive centre for Italian glass production. According to Rosa Barovier Mentasti, a glass historian and descendant of the Barovier family of glass-makers, aventurine was one of the most ambitious of the Murano artisans’ secret techniques. Even during the crisis that hit the glass-making industry in the 18th century (due to the growth of foreign competition), aventurine and other techniques including calcedonio did not feel the consequences of foreign trade, as they were seen as prized Venetian specialities.
The preparation is long and delicate, and its success so difficult to achieve that it was said to be an adventure, or avventura in Italian. Might this be another source of the name?
A transformative experience: copper oxide to metallic copper
Aventurine has a special ingredient that causes its characteristic crystal ‘stars’ to develop: copper oxide. This unassuming black powder is added to hot, malleable glass, and is reduced to metallic copper in the crucible. Reduction is the chemical process by which oxygen is removed, therefore copper oxide (CuO) becomes elemental copper (Cu). A reducing atmosphere can be created by burning organic materials such as wood in the furnace to produce smoke. Alternatively, the atmosphere can be achieved by adding compounds including oxides of iron, tin, antimony and even durum wheat. These reducing agents are added to the molten glass in small sachets called scartocci. Once reduced, the metallic copper particles precipitate throughout the glass in crystalline structures which, when viewed under a microscope, reveal themselves to be triangular and hexagonal in shape. Seen with intense magnification, the crystals appear to be suspended in a colourless vitreous solid.
Recent research shows that the chief difficulty in producing aventurine is not in the reduction or the nucleation (the moment at which particles begin to appear within the vitreous mass), but in the enlargement and dispersion of the copper crystals. This process occurs during the very slow cooling period. The point at which the cooling process begins is of paramount importance when making aventurine. The glass must be cooled slowly until it reaches ambient temperature, and it is during this time that the crystals grow, becoming almost visible to the naked eye.
Bringing it all together: Adventurine and Chalcedony
In order to incorporate aventurine into chalcedony glass, another process must take place. Once a block of aventurine is produced it is crushed coarsely into small fragments. These are placed near the mouth of the furnace so that they nearly soften. The chalcedony glass is prepared onto a blowpipe, and rolled over the pre-heated aventurine, which sticks to the chalcedony glass. A technique called marvering is used: the molten glass is rolled against a hard, flat surface to cause the aventurine to become flush with the surface of the glass. The aventurine inclusions become ovals through the subsequent glass-blowing process, which inflates the form and elongates the aventurine into its final shape.
A ‘Sweet Contest of Nature and Man’
Renaissance Italians marvelled at naturally-occurring stones and minerals, such as chalcedony and agate quartz, for their swirling, variegated, patterns of colour. This sense of wonder was part of a wider fascination with natural phenomena in the 16th century. Curiosity cabinets or Wunderkammer of the period routinely contained examples of natural stones. These could be presented in a variety of ways and were often cut, polished and set with golden mounts. The stones would keep company with an eclectic array of items from the natural world, from skeletons and stuffed animals to seashells and corals.

The popularity of these precious natural stones propelled the glass industry towards devising convincing substitutes. As such, craftsmen could tap into the demand for natural wonders at a competitive price. The famous glass-makers of Murano were successful in this quest. In his De situ Venetae urbis (‘The Position of the Venetian City’), of c. 1495, the Venetian scholar Marcantonio Coccio Sabellico (1436–1506) wrote that ‘there is no kind of precious stone that cannot be imitated by the industry of glass-workers, a sweet contest of nature and man.’
The hard work that went into creating imitations of nature demonstrated to Renaissance buyers the skill, intelligence and artistry that humankind could achieve. It would have surely seemed like magic to see glass resembling stone coming out of the workshops. Antonio Neri’s L’Arte Vetraria (‘The Art of Glass’), a collection of Venetian glass-making recipes from 1612, describes the beauty of a piece of glasswork in the following terms: ‘it will seem as if nature herself could not arrive at such perfection, nor art imitate it’.

The added value of the human touch

Calcedonio glass, named after chalcedony quartz, is an example of glass made to imitate minerals. Its swirling patterns and brown, murky colours are reminiscent of hard, polished stone. To achieve such an aesthetically pleasing final product requires a labour-intensive process. Metal oxides, such as silver, tin, iron, copper and mercury, are added to the molten transparent glass base, and are roughly mixed to create a marbled effect. It may seem ironic that such a difficult and labour-intensive process should be required to imitate stones which exist in the natural world, but glassware is continually available, unlike stone which must be mined, and can therefore meet demand much more quickly. Particularly in the time of the Renaissance artisans, the skilled craftsmanship itself added to the value of glass objects and made them all the more treasured. The high price demanded by chalcedony glass can be seen in a commission of eleven chalcedony vases, made in 1475 by the great Florentine banker and statesman Filippo Strozzi the Elder (1428–91), who paid the handsome figure of 55 ducats.
Long before major industrial or technological revolutions, nature was the producer of all that was beautiful and perfect in the world. It was a marvel to own something man-made that rivalled the previously incomparable beauty of the natural world, as it demonstrated the supremacy and skill of human ability.
Aventurra: Chance and Serendipity in Scientific Discovery and Glass-making
In all its technical complexity, can it really be true that aventurine is the product of a happy accident? Science, unlike creativity in the arts, is popularly viewed as being practised through precise and thoughtfully prepared acts. Hypotheses are made, before experiments are planned in minute detail. The steps are then followed exactly as the methodology has specified, until a result is achieved. Each step is of critical importance. In the case of aventurine glass, art appears to reign supreme over science, as the visual effect is believed to have blossomed first from a fortunate mistake. Perhaps a scientific approach would not have resulted in the creation of such beautiful and prized glass.
However, science is not always as accurately predicted as some might think. Fortunate mistakes, happy accidents, or serendipity have played a large part in some of the most important scientific discoveries ever made. From Archimedes’ (288–12 BCE) bath and Newton’s (1642–1727) apple tree to Pfizer’s surprise discovery of Viagra, there are countless examples of serendipity in science throughout the ages.
The Penicillin precedent

In 1928, Alexander Fleming’s (1881–1955) serendipitous discovery changed modern medicine forever. Although a brilliant and respected researcher, his laboratory was often untidy. When he looked at the petri dishes he had stored away over the summer, perhaps like the first person to discover golden pools of aventurine in their glass, he was confounded. ‘That’s funny’, he famously remarked. The dishes had become contaminated with a fungus, and where this fungus grew, staphylococci had been killed. Fleming isolated the fungus and grew it, noting that it exuded a substance which had the ability to kill bacteria. Fleming named his discovery penicillin.
‘When I woke up just after dawn on September 28, 1928, I certainly didn’t plan to revolutionise all medicine by discovering the world’s first antibiotic, or bacteria killer, but I suppose that was exactly what I did.‘
Selective serendipity
To suggest that such a monumental scientific discovery as antibiotics happened only by chance could be insulting to the great minds that grappled with the task. However, we might turn our attention to Horace Walpole (1717–97), the celebrated 18th-century man of letters. Walpole coined the term serendipity, and in so doing specified that the word referred to a particular kind of happy accident, one which only a sagacious — or clever — person can exploit. The basis of ‘serendipity’ came from a Persian fairytale that Walpole had heard, entitled ‘The Three Princes of Serendip’. The princes, by ‘accidents and sagacity’, manage to identify a lost camel having never seen it, through inferring aspects of its appearance from descriptions of its experiences. ‘Serendip’ here referred to Sri Lanka.
Louis Pasteur (1822–95), whose discovery of the chicken cholera vaccine is sometimes cited as an example of serendipity, similarly stated that ‘chance favours only the prepared mind’.
Even if the first glass-maker to discover aventurine did so by knocking over a plate of copper oxide into the molten glass, it is only through generations of Muranese expertise (Walpole’s ‘sagacity’) that this accident was harnessed and refined to create the beautiful wares known as aventurine.
Acknowledgments
I am grateful to the following scholars for their knowledgeable insight and help:
William Gudenrath, Bernard Gratuze, Suzanne Higgott, and Marco Verità.
Bibliography
Barovier Mentasti, Rosa. Glass in Murano. Venice: Veneto Region and the Venice Chamber of Commerce, 1983.
Colman, David R. ‘The three princes of Serendip: Notes on a mysterious phenomenon’. McGill Journal of Medicine, Vol. 9, No. 2 (July, 2006): 161–163.
Dunbar, Kevin Niall, and Jonathan A. Fugelsang. ‘Causal thinking in science: How scientists and students interpret the unexpected’. In Scientific and Technological Thinking, edited by 57–79. Mahwah, New Jersey: Lawrence Erlbaum Associates, 2004.
Edwards, Geoffrey R., and Garry Sommerfeld. Art of Glass: Glass in the Collection of the National Gallery of Victoria. Melbourne: National Gallery of Victoria, 1998.
Higgott, Suzanne. Wallace Collection Catalogues: Glass and Limoges Painted Enamels. London: Trustees of The Wallace Collection, 2011.
Moretti, Cesare, Bernard Gratuze and Sandro Hreglich. ‘Le verre aventurine (“avventurina”): son histoire, les recettes, les analyses, sa fabrication’. ArchéoSciences, No. 37 (2013): 135–54.
Neri, Antonio. L’Arte vetraria. Florence: Stamperia de’Giunti, 1612.
Sabellico, Marcantonio Coccio, De situ urbis Venetae. c. 1495.
Washington, Henry S. ‘On Copper Crystals in Aventurine Glass’. American Journal of Science, Vol. 3, No. 48 (1894): 287, 411–18.