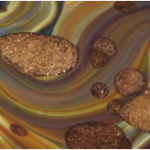
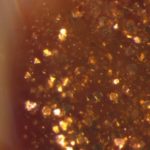
Aventurine is the name given to a Venetian glassmaking technique developed in 16th century Murano, at the heart of the Italian glassmaking tradition. Aventurine from this period is seen as ‘inclusions’ of golden sparkles – a vitreous mass characterised by small shining copper crystals in the glass. The reflective copper crystals twinkle and are called ‘stelle’ or ‘stars’, which gives aventurine its sometimes-used second name, stellaria.
Murano’s Edge
According to Rosa Barovier Mentasti, a glass historian and descendent of the Barovier family of glassmakers, aventurine was one of the most ambitious of the Murano glassmakers’ secret techniques. Even during the crisis that hit the glassmaking industry in the 18th century (due to the growth of foreign competition), aventurine and other techniques including calcedonio, did not feel the consequences of foreign trade, as they were seen as prized Venetian specialities.
The preparation is long and delicate, and its success so difficult to achieve that it was said to be an adventure – avventura in Italian – a possible source for the name aventurine.
A transformative experience: copper oxide to metallic copper
Aventurine has a special ingredient that causes its characteristic crystal ‘stars’ to develop: copper oxide. This unassuming black powder is added to hot, malleable glass, and is reduced to metallic copper in the crucible. Reduction is the chemical process by which oxygen is removed, therefore copper oxide (CuO) becomes elemental copper (Cu). A reducing atmosphere can be created by burning organic materials, such as wood, in the furnace to produce smoke, or by adding compounds including oxides of iron, tin and antimony and even durum wheat. These reducing agents were added to the molten glass in small sachets called scartocci. Once reduced, the metallic copper particles precipitate throughout the glass in crystalline structures, which, when viewed under a microscope reveal themselves to be triangular and hexagonal in shape. Under a microscope, the crystals appear to be suspended in a colourless vitreous matrix.
Producing the copper crystals
Recent research shows that the chief difficulty in producing aventurine is not in the reduction or the nucleation (the moment at which particles begin to appear within the vitreous mass), but is in the enlargement and dispersion of the copper crystals. This process occurs during the very slow cooling period. The point at which the cooling process is begun is of high importance in the success of making aventurine. The glass must be cooled slowly until it reaches ambient temperature, and it is during this time that the crystals grow, becoming almost visible to the naked eye.
Bringing it all together: Adventurine and Chalcedony
In order to incorporate aventurine into chalcedony glass, another process must take place. Once a block of aventurine is produced it is crushed coarsely into small fragments. These are placed near the mouth of the furnace so that they nearly soften. The chalcedony glass is prepared onto a blowpipe, and rolled over the pre-heated aventurine, which sticks to the chalcedony glass. A technique called marvering is used – the molten glass is rolled against a hard, flat surface to cause the aventurine to become flush with the surface of the glass. The aventurine inclusions become ovals through the subsequent glassblowing process: inflating, elongating and torqueing pull the aventurine into its final shape.
Back to A Venetian Chalcedony and Adventurine Glass Bowl
Acknowledgments
I am grateful to the following scholars for their knowledgeable insight and help:
William Gudenrath, Bernard Gratuze, Suzanne Higgott, and Marco Verità.
Bibliography
Barovier Mentasti, R. (1983) Glass in Murano. Venice: Veneto Region and the Venice Chamber of Commerce.
Moretti, C. et al (2013) Le verre aventurine (“avventurina”): son histoire, les recettes, les analyses, sa fabrication. ArchéoSciences, 37: 2013, pp. 135-154
Washington, H. (1894) On Copper Crystals in Aventurine Glass. American Journal of Science. 3rd series, 48: 287, pp. 411-418